How to Apply

How to Apply
Overview
QPHI is launching its new Research Portal which will give an opportunity to the researcher to submit access application forms online. Please use this link to register and submit your proposal.
Research Portal
To access the QPHI Research Portal, click here.
The data and Bio samples collected or generated by QPHI will be made available to researchers employed within or otherwise contractually bound to public and private institutions that conduct scientific research and that meet the requirements detailed in the QPHI Research Access policy. Approved Users will be given access to QPHI Research Data and/or Bio samples for the period agreed upon in the approved Access Agreement, with the possibility of subsequent renewal.
All researchers interested in applying are required to create a profile through the Research Portal where further information and the application form is available.
Should you have any queries about sample availability or data type, a pre-application query form is available within the research portal.

Research Portal Support
For any support requests, please use this email: qphisupport@qf.org.qa
Click here to download a pdf version.

Application Forms
- QPHI RESEARCH PROGRESS REPORT
- QPHI GENOMIC ANALYSIS RETRIEVAL FORM
- QPHI SUPPLEMENTARY APPLICATION FORM
- QPHI RESEARCH APPEAL REQUEST
- QPHI RESEARCH CLAIM FORM
- QPHI REMOTE DATA ACCESS AGREEMENT
- QPHI REMOTE ACCESS REQUEST FORM
- QPHI IRB AMENDMENT FORM
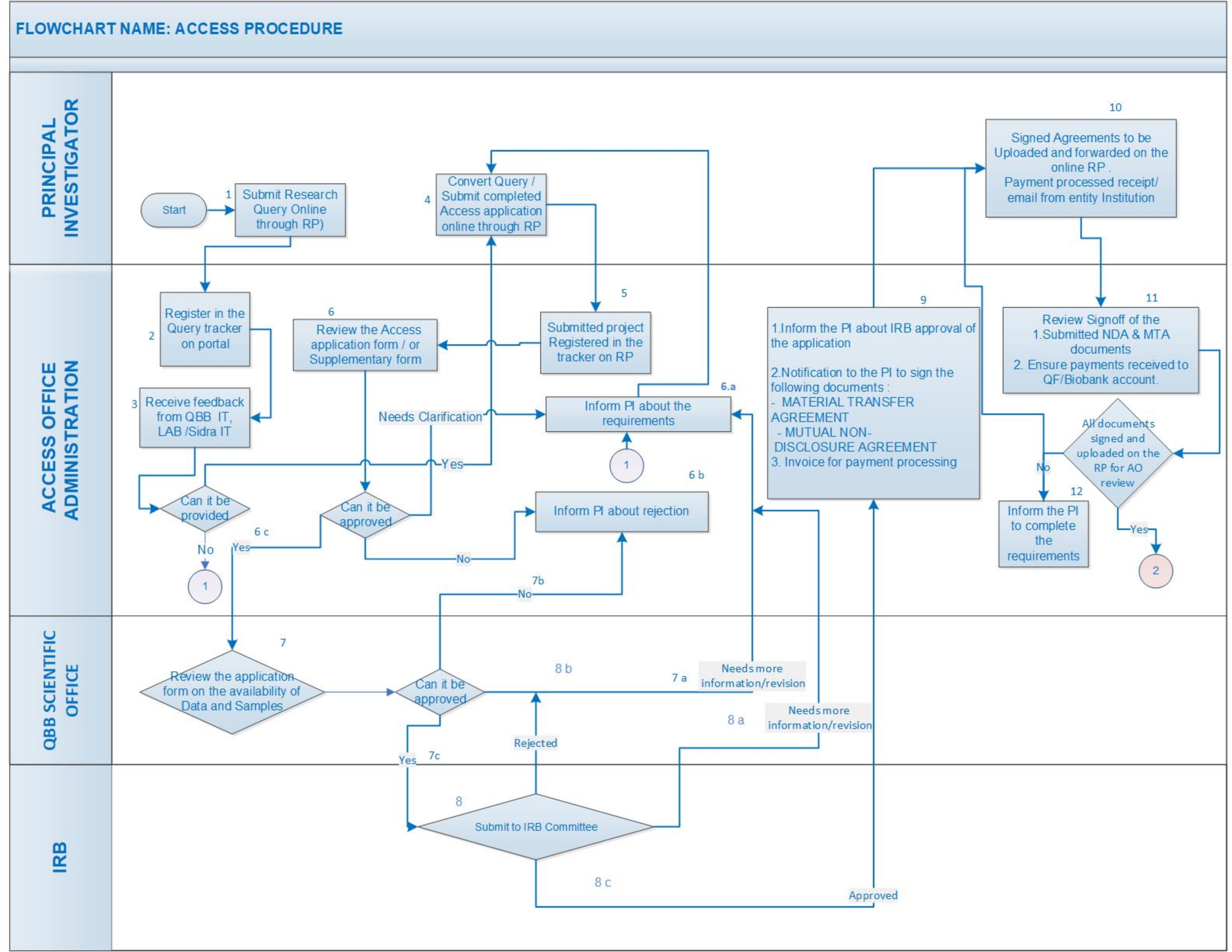
Flow chart narrative
Important: Add qphi.alerts@qf.org.qa email address to your contact so you can receive emails in your inbox and not in the spam or junk folder.
- Create a profile/login here.
- Complete and submit the Research Access Application Form. Data and Bio Samples prices are available here. Latest prices are updated on the Portal.
- Application form received and reviewed by QPHI
- Application sent for IRB review or rejected
- Rejected applications may be eligible for an appeals process
QPHI Institutional Review Board (IRB) Committee
All research applications involving human research subjects will require submission and approval from the QPHI IRB committee. The QPHI IRB committee was founded on the 30th of September 2016.
The purpose of the QPHI IRB committee is to formalize the process of medical research work of Qatar Precision Health Institute which it is committed to and ensuring that all human subject research in which it is engaged is conducted in accordance with the ethical principles through an Institutional Review Board Committee (IRB) as stated by Supreme Council of Health in Qatar: Policies, Regulations and Guidelines for Research Involving Human 2009.
Qatar Precision Health Institute IRB Committee will:
Review the appropriateness of the research protocol as well as the risks and benefits to study participant information. It will ensure that clinical trial participant information is exposed to minimal risks in relation to any benefits that might result from the research.
Review and approve all study-related materials before and during the research.
Assist in biomedical research horizon scanning to help Qatar Precision Health Institute prepare for and adapt to emerging trends in biomedical research, new technologies and new opportunities to contribute to the advancement of medical research

